Isomers are when two molecules have exactly the same atoms, but those atoms are put together in different ways
Resonance occurs when one particular molecule has multiple ways in which the electrons will fit into the compound. For example:
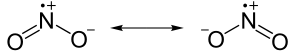
In this compound, the Nitrogen can either be double bounded with the oxygen on the right or the oxygen on the left. This is not a slight of hand. I am not flipping the molecule over like a pancake and turning the front side into the back side or the left into the right. The oxygen on the left in the first picture is the same oxygen on the left in the second picture. This is true because the octet rule for lewis structures makes both recordings equally as accurate.
In these situations, where multiple lewis structures are applicable, the thought is that electrons want to be in two different places equally as much. And therefore these electrons become delocalized. It is believed that these electrons are not simply moving back and forth between different atoms, but are actually in some kind of hybrid state.
Take the ozone molecule O3 for example:
 |
| image obtained from wikipedia |
Because the lewis dot and kekule structures cannot accurately depict the resonance molecule, the hybrid is generated.
Each lewis structure is considered a contributor to the hybrid, but the hybrid is the most accurate depiction of the reality of how electrons are being used to bond atoms together.
What's the most famous resonance hybrid structure? I'm thinking Benzene. What are your thoughts?
No comments:
Post a Comment